

过程工程学报 ›› 2021, Vol. 21 ›› Issue (7): 807-816.DOI: 10.12034/j.issn.1009-606X.220217CSTR: 32067.14.jproeng.220217
李凌1( ), 丁磊1,2(
), 丁磊1,2( ), 薛岗1, 贾韫翰1, 钟梅英1,2, 张德伟2,3
), 薛岗1, 贾韫翰1, 钟梅英1,2, 张德伟2,3
收稿日期:2020-07-09
修回日期:2020-09-04
出版日期:2021-07-28
发布日期:2021-07-27
通讯作者:
丁磊 17375087687@163.com;dinglei1978@163.com
作者简介:李凌(1997-),男,安徽省合肥市人,硕士研究生,建筑与土木工程专业,E-mail: 17375087687@163.com基金资助:
LI Ling1( ), Lei DING1,2(
), Lei DING1,2( ), Gang XUE1, Yunhan JIA1, Meiying ZHONG1,2, Dewei ZHANG2,3
), Gang XUE1, Yunhan JIA1, Meiying ZHONG1,2, Dewei ZHANG2,3
Received:2020-07-09
Revised:2020-09-04
Online:2021-07-28
Published:2021-07-27
Contact:
Lei DING 17375087687@163.com;dinglei1978@163.com
摘要:
研究了不同亲疏水性腐殖酸对磁性离子交换(MIEX)树脂吸附去除溴离子的影响。溶液pH=7.0条件下,四种腐殖酸组分(强疏水性、弱疏水性、极性亲水、中性亲水)对溴离子的去除表现出不同程度的抑制作用。相较而言疏水性组分的不利影响较为显著。腐殖酸的存在减弱了溴离子在MIEX树脂上吸附过程对pH值的依赖性。腐殖酸组分能加速溴离子的吸附速率,溴离子在树脂上达到吸附平衡所需的时间被显著缩短。无论溶液中是否存在腐殖酸,拟二级动力学模型均能很好地拟合溴离子在树脂上的吸附过程,并且由于竞争吸附作用,腐殖酸组分导致溴离子在MIEX树脂上的平衡吸附容量显著减少。溴离子在MIEX树脂上的吸附平衡均可以通过Langmuir和Freundlich模型进行拟合。腐殖酸组分的存在会降低溴离子吸附体系的自发性,强疏水性组分的影响较为显著。该研究结果对于有效控制水源中溴离子具有重要意义。
中图分类号:
李凌, 丁磊, 薛岗, 贾韫翰, 钟梅英, 张德伟. 不同亲疏水性腐殖酸对磁性离子交换树脂吸附去除水中溴离子的影响[J]. 过程工程学报, 2021, 21(7): 807-816.
LI Ling, Lei DING, Gang XUE, Yunhan JIA, Meiying ZHONG, Dewei ZHANG. Effects of hydrophilicity/hydrophobicity of humic acid components on the removal of bromide adsorbed on magnetic ion exchange resin[J]. The Chinese Journal of Process Engineering, 2021, 21(7): 807-816.
| Parameter | MIEX |
|---|---|
| Type | Macroporous |
| Structure | Polyacrylic |
| Zero charge point/MW | 6.4 |
| Particle size/mm | 0.15 |
| Water content/% | 65~67.1 |
| Magnetic material content/% | 8.52 |
| Total pore volume/(cm3/mL) | 0.018 |
| Exchange capacity/(meq/mL) | 0.32~0.65 |
| BET specific surface area/(m2/g) | 21.47 |
表1 MIEX树脂的性能参数[22]
Table 1 Performance parameters of MIEX resin[22]
| Parameter | MIEX |
|---|---|
| Type | Macroporous |
| Structure | Polyacrylic |
| Zero charge point/MW | 6.4 |
| Particle size/mm | 0.15 |
| Water content/% | 65~67.1 |
| Magnetic material content/% | 8.52 |
| Total pore volume/(cm3/mL) | 0.018 |
| Exchange capacity/(meq/mL) | 0.32~0.65 |
| BET specific surface area/(m2/g) | 21.47 |
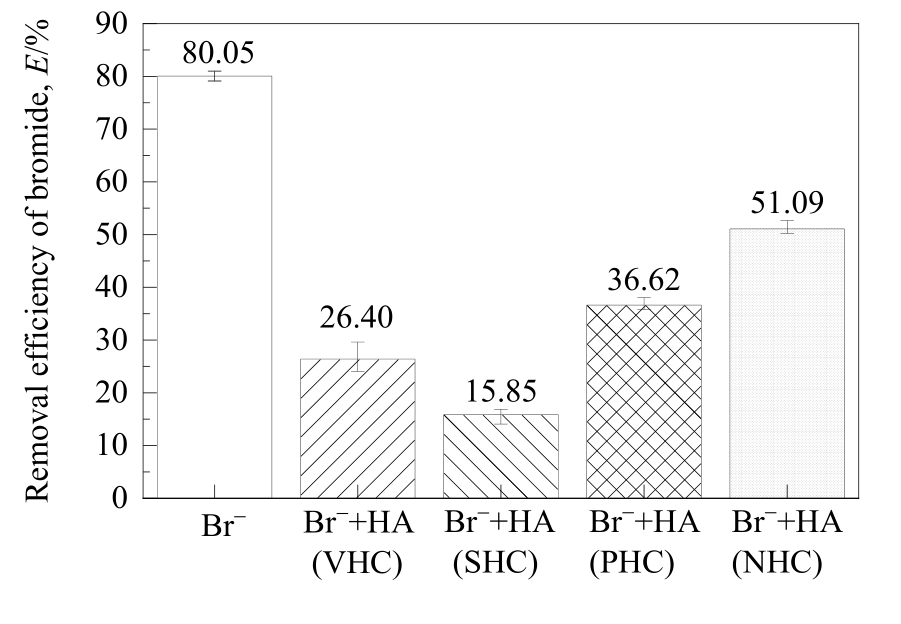
图2 不同亲/疏水性HA组分对MIEX树脂吸附溴离子的影响
Fig.2 Effect of HA fractions with different hydrophilicity/hydrophobicity on the removal of bromide adsorbed on MIEX resin
| Zeta potential/mV | Component of HA | |||
|---|---|---|---|---|
| VHC | SHC | PHC | NHC | |
| pH=7.0 | -12.70 | -12.60 | -9.54 | -8.78 |
表2 四种HA组分的Zeta电位
Table 2 The Zeta potentials of four HA components
| Zeta potential/mV | Component of HA | |||
|---|---|---|---|---|
| VHC | SHC | PHC | NHC | |
| pH=7.0 | -12.70 | -12.60 | -9.54 | -8.78 |
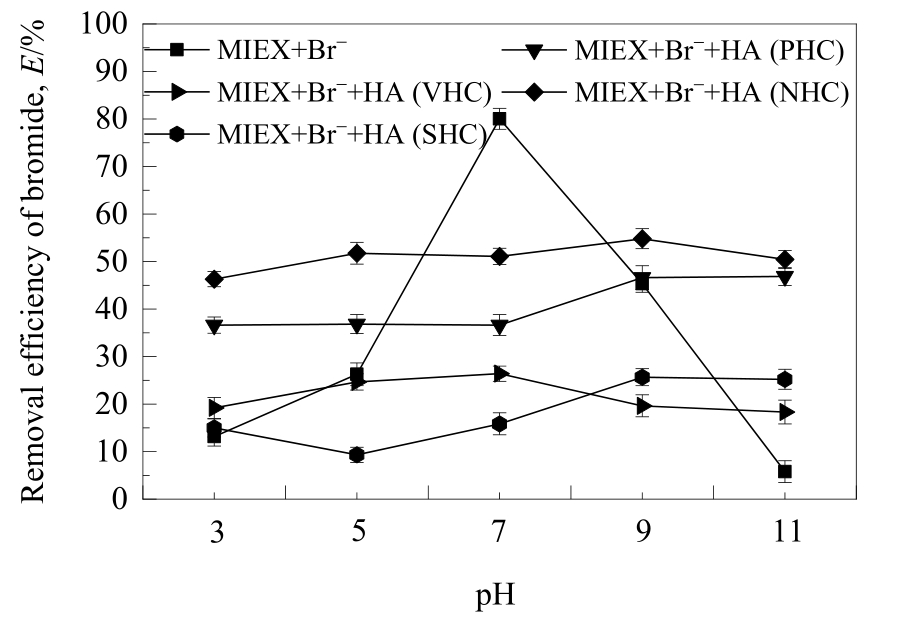
图4 不同亲/疏水性HA组分在不同pH值下对MIEX树脂吸附溴离子的影响
Fig.4 Effect of HA fractions with different hydrophilicity/hydrophobicity on the removal of bromide adsorbed on MIEX resin at different pH values
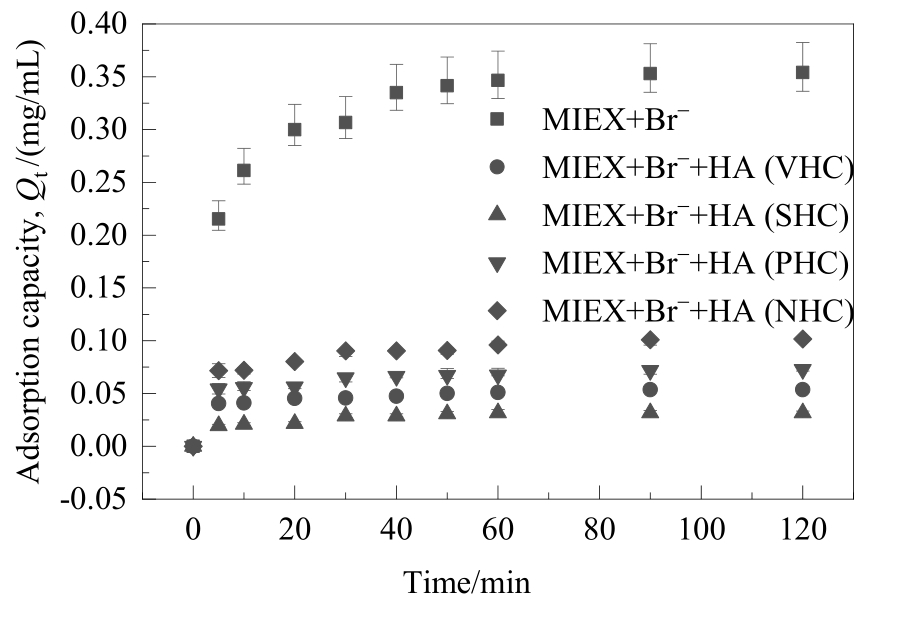
图5 不同亲/疏水性HA组分对溴离子在MIEX树脂上吸附动力学的影响
Fig.5 Effect of HA fractions with different hydrophilicity/hydrophobicity on the kinetics of bromide adsorbed on MIEX resin
| Component of bromide solution | Pseudo-first order kinetic model | Pseudo-second order kinetic model | ||||||
|---|---|---|---|---|---|---|---|---|
qe/ (mg/L) | k1/min-1 | R2 | SE | qe/ (mg/L) | k2/min-1 | R2 | SE | |
| Br- | 0.34 | 0.170 | 0.94 | 0.008 | 0.37 | 0.710 | 0.99 | 0.005 |
| Br-+HA (VHC) | 0.05 | 0.294 | 0.95 | 0.001 | 0.05 | 10.056 | 0.98 | 0.001 |
| Br-+HA (SHC) | 0.03 | 0.135 | 0.91 | 0.001 | 0.03 | 6.158 | 0.96 | 0.001 |
| Br-+HA (PHC) | 0.07 | 0.292 | 0.94 | 0.002 | 0.07 | 7.150 | 0.97 | 0.002 |
| Br-+HA (NHC) | 0.09 | 0.229 | 0.94 | 0.003 | 0.10 | 3.761 | 0.98 | 0.003 |
表3 吸附动力学模型计算的参数
Table 3 Parameters calculated from adsorption kinetics model
| Component of bromide solution | Pseudo-first order kinetic model | Pseudo-second order kinetic model | ||||||
|---|---|---|---|---|---|---|---|---|
qe/ (mg/L) | k1/min-1 | R2 | SE | qe/ (mg/L) | k2/min-1 | R2 | SE | |
| Br- | 0.34 | 0.170 | 0.94 | 0.008 | 0.37 | 0.710 | 0.99 | 0.005 |
| Br-+HA (VHC) | 0.05 | 0.294 | 0.95 | 0.001 | 0.05 | 10.056 | 0.98 | 0.001 |
| Br-+HA (SHC) | 0.03 | 0.135 | 0.91 | 0.001 | 0.03 | 6.158 | 0.96 | 0.001 |
| Br-+HA (PHC) | 0.07 | 0.292 | 0.94 | 0.002 | 0.07 | 7.150 | 0.97 | 0.002 |
| Br-+HA (NHC) | 0.09 | 0.229 | 0.94 | 0.003 | 0.10 | 3.761 | 0.98 | 0.003 |
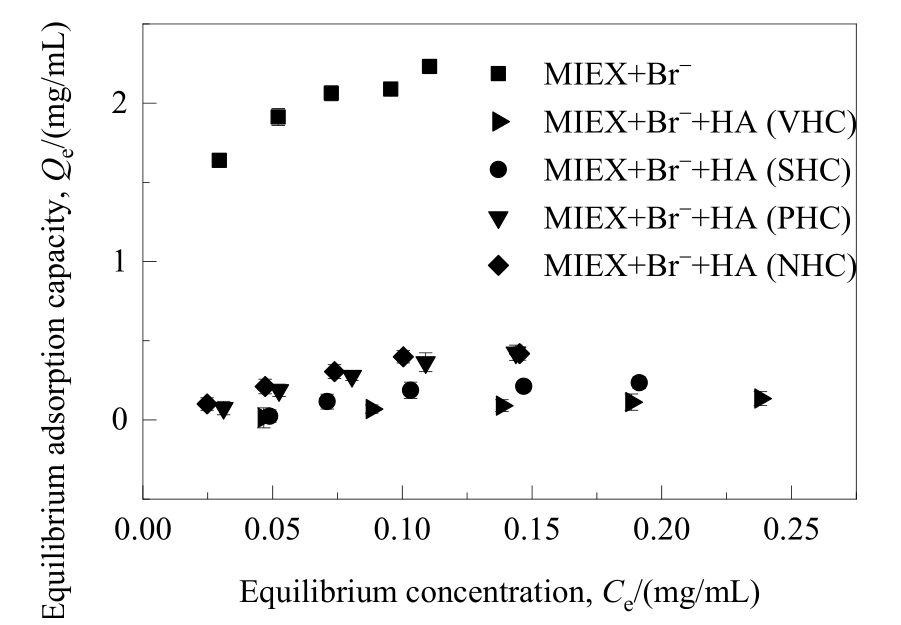
图6 不同亲/疏水性HA组分对溴离子在MIEX树脂上吸附平衡的影响
Fig.6 Effect of HA fractions with different hydrophilicity/hydrophobicity on the adsorption equilibrium of bromide adsorbed on MIEX resin
Component of bromide solution | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| qmax/(mg/mL) | kl/(L/mg) | R2 | SE | kf | 1/n | R2 | SE | |
| Br- | 13.10 | 0.001 | 0.99 | 0.307 | 2.50 | 0.853 | 0.99 | 0.020 |
| Br-+HA (VHC) | 1.04 | 0.304 | 0.96 | 0.630 | 0.27 | 1.046 | 0.95 | 0.186 |
| Br-+HA (SHC) | 1.10 | 0.578 | 0.93 | 0.906 | 0.47 | 1.101 | 0.92 | 0.259 |
| Br-+HA (PHC) | 1.73 | 0.052 | 0.95 | 0.582 | 0.91 | 0.968 | 0.95 | 0.171 |
| Br-+HA (NHC) | 1.40 | 0.913 | 0.98 | 0.517 | 0.78 | 1.237 | 0.97 | 0.152 |
表4 吸附平衡模型计算的参数
Table 4 Parameters calculated from adsorption equilibrium model
Component of bromide solution | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| qmax/(mg/mL) | kl/(L/mg) | R2 | SE | kf | 1/n | R2 | SE | |
| Br- | 13.10 | 0.001 | 0.99 | 0.307 | 2.50 | 0.853 | 0.99 | 0.020 |
| Br-+HA (VHC) | 1.04 | 0.304 | 0.96 | 0.630 | 0.27 | 1.046 | 0.95 | 0.186 |
| Br-+HA (SHC) | 1.10 | 0.578 | 0.93 | 0.906 | 0.47 | 1.101 | 0.92 | 0.259 |
| Br-+HA (PHC) | 1.73 | 0.052 | 0.95 | 0.582 | 0.91 | 0.968 | 0.95 | 0.171 |
| Br-+HA (NHC) | 1.40 | 0.913 | 0.98 | 0.517 | 0.78 | 1.237 | 0.97 | 0.152 |
| Component | T/K | KD/(L/mL) | ΔG0/ (kJ/mol) | ΔH0/ (kJ/mol) | ΔS0/(J/mol) |
|---|---|---|---|---|---|
| Br- | 288 | 18.37 | -6.77 | -92.40 | -279.33 |
| 293 | 7.35 | -5.28 | |||
| 298 | 5.05 | -3.79 | |||
| Br-+VHC | 288 | 0.63 | 1.10 | 18.45 | 60.24 |
| 293 | 0.74 | 0.80 | |||
| 298 | 0.81 | 0.50 | |||
| Br-+SHC | 288 | 1.23 | -0.56 | 2.16 | 9.44 |
| 293 | 1.37 | -0.61 | |||
| 298 | 1.26 | -0.65 | |||
| Br-+PHC | 288 | 3.04 | -2.83 | -20.23 | -60.40 |
| 293 | 3.27 | -2.53 | |||
| 298 | 2.29 | -2.23 | |||
| Br-+NHC | 288 | 2.89 | -2.70 | -21.89 | -66.61 |
| 293 | 3.00 | -2.37 | |||
| 298 | 2.12 | -2.04 |
表5 HA组分存在时Br-在MIEX树脂上的吸附热力学参数
Table 5 Thermodynamic parameters of Br- absorbed on MIEX resin in the presence of HA component
| Component | T/K | KD/(L/mL) | ΔG0/ (kJ/mol) | ΔH0/ (kJ/mol) | ΔS0/(J/mol) |
|---|---|---|---|---|---|
| Br- | 288 | 18.37 | -6.77 | -92.40 | -279.33 |
| 293 | 7.35 | -5.28 | |||
| 298 | 5.05 | -3.79 | |||
| Br-+VHC | 288 | 0.63 | 1.10 | 18.45 | 60.24 |
| 293 | 0.74 | 0.80 | |||
| 298 | 0.81 | 0.50 | |||
| Br-+SHC | 288 | 1.23 | -0.56 | 2.16 | 9.44 |
| 293 | 1.37 | -0.61 | |||
| 298 | 1.26 | -0.65 | |||
| Br-+PHC | 288 | 3.04 | -2.83 | -20.23 | -60.40 |
| 293 | 3.27 | -2.53 | |||
| 298 | 2.29 | -2.23 | |||
| Br-+NHC | 288 | 2.89 | -2.70 | -21.89 | -66.61 |
| 293 | 3.00 | -2.37 | |||
| 298 | 2.12 | -2.04 |
| 1 | Lü L, Wang Y, Wei M, et al. Bromide ion removal from contaminated water by calcined and uncalcined MgAlCO3 layered double hydroxides [J]. Journal of Hazardous Materials, 2008, 152(3): 1130-1137. |
| 2 | Zhang H F, Yang M. Characterization of brominated disinfection byproducts formed during chloramination of fulvic acid in the presence of bromide [J]. Science of the Total Environment, 2018, 627: 118-124. |
| 3 | Xu Z Z, Jian R Y, Yan X M, et al. Competitive removal of DOM and bromide in raw waters by MIEX and iron coagulation [J]. Water Supply, 2013, 13(1): 123-129. |
| 4 | 牛志广, 张玉彬, 吕志伟, 等. 溴离子对预氯化和常规水处理工艺中消毒副产物的影响 [J]. 西南师范大学学报(自然科学版), 2019, 44(1): 109-117. |
| Niu Z G, Zhang Y B, Lü Z W, et al. Effect of bromine on disinfection by-products in prechlorination and conventional water treatment process [J]. Journal of Southwest China Normal University (Natural Science Edition), 2019, 44(1): 109-117. | |
| 5 | 梅红, 丁国际, 黄鑫, 等. 含溴黄浦江水消毒过程中溴代三卤甲烷和卤乙酸的生成特性 [J]. 环境科学学报, 2011, 31(10): 2162-2168. |
| Mei H, Ding G J, Huang X, et al. Formation of Br-THMs and Br-HAAs in bromide-containing Huangpu river water during disinfection [J]. Acta Scientiae Circumstantiae, 2011, 31(10): 2162-2168. | |
| 6 | 孟欣, 魏彬, 李学艳, 等. 溴离子对三卤甲烷生成量的影响因素研究 [J]. 中国给水排水, 2019, 35(19): 66-71. |
| Meng X, Wei B, Li X Y, et al. Factors affecting the formation of trihalomethanes by bromide ions [J]. China Water & Wastewater, 2019, 35(19): 66-71. | |
| 7 | 李明阳. 烹饪过程中氯代溴代碘代类三卤甲烷生成的影响因素 [J]. 净水技术, 2018, 37(6): 21-28. |
| Li M Y. Influencing factors of formation of Cl/Br/I-THMs under simulated cooking conditions [J]. Water Purification Technology, 2018, 37(6): 21-28. | |
| 8 | 鲁金凤, 王楚亚, 刘宇心, 等. 活性炭去除饮用水中溴酸盐的研究进展 [J]. 水资源与水工程学报, 2013, 24(5): 6-10, 16. |
| Lu J F, Wang C Y, Liu Y X, et al. Review on bromate removal from drinking water by using active carbon [J]. Journal of Water Resources & Water Engineering, 2013, 24(5): 6-10, 16. | |
| 9 | Wang Y J, Yu J W, Han P, et al. Advanced oxidation of bromide-containing drinking water: a balance between bromate and trihalomethane formation control [J]. Journal of Environmental Sciences, 2013, 25(11): 2169-2176. |
| 10 | 董晓晨, 阮春蓉, 陈子华, 等. 磁性离子交换树脂(MIEX®)去除原水中有机物的实验 [J]. 净水技术, 2018, 37(9): 15-18, 31. |
| Dong X C, Ruan C R, Chen Z H, et al. Removal of organic matter in raw water by magnetic ion exchange (MIEX®) resin [J]. Water Purification Technology, 2018, 37(9): 15-18, 31. | |
| 11 | 贾韫翰, 丁磊, 任培月, 等. 基于响应曲面法的磁性离子交换树脂去除甲基橙和刚果红的优化 [J]. 过程工程学报, 2020, 20(9): 1035-1044. |
| Jia Y H, Ding L, Ren P Y, et al. Removal optimization of methyl orange and Congo red adsorbed on MIEX resin using response surface methodology [J]. The Chinese Journal of Process Engineering, 2020, 20(9): 1035-1044. | |
| 12 | 王冠宁, 王亮, 张朝晖, 等. 磁性阴离子交换树脂(MIEX®)再生工艺的研究 [J]. 工业水处理, 2015, 35(8): 37-41. |
| Wang G N, Wang L, Zhang C H, et al. Research on the regeneration process of magnetic anion exchange resin (MIEX®) [J]. Industrial Water Treatment, 2015, 35(8): 37-41. | |
| 13 | Hsu S, Singer P C. Removal of bromide and natural organic matter by anion exchange [J]. Water Research, 2010, 44(7): 2133-2140. |
| 14 | Walker K M, Boyer T H. Long-term performance of bicarbonate-form anion exchange: removal of dissolved organic matter and bromide from the St. Johns River, FL, USA [J]. Water Research, 2011, 45(9): 2875-2886. |
| 15 | Singer P C, Schneider M, Edwards-brandt J, et al. MIEX for removal of DBP precursors: pilot-plant findings [J]. American Water Works Association Journal, 2007, 99(4): 128-139. |
| 16 | Chen Wei, Cao Zhe, Liu Chen, et al. Removal efficiency and influence factors of bromide in water by MIEX [J]. Journal of Civil Architectural & Environmental Engineering, 2012, 34(3): 133-137. |
| 17 | 王文东, 张轲, 范庆海, 等. 紫外辐射对腐殖酸溶液理化性质及其混凝性能的影响 [J]. 环境科学, 2016, 37(3): 994-999. |
| Wang W D, Zhang K, Fan Q H, et al. Effects of UV radiation on the physicochemical properties and coagulation properties of humic acid solution [J]. Environmental Science, 2016, 37(3): 994-999. | |
| 18 | 刘诗婷, 么强, 陈芳, 等. 腐殖酸联合铁氧化物去除水体中重金属的研究进展 [J]. 工业水处理, 2020, 40(5): 7-11. |
| Liu S T, Me Q, Chen F, et al. Research progress on removal of heavy metals from water by humic acid combined with iron oxide [J]. Industrial Water Treatment. 2020, 40(5): 7-11. | |
| 19 | Avena M J, Koopal L K. Kinetics of humic acid adsorption at solid-water interfaces [J]. Environmental Science & Technology, 1999, 33(16): 2739-2744. |
| 20 | Phetrak A, Lohwacharin J, Takizawa S. Analysis of trihalomethane precursor removal from sub-tropical reservoir waters by a magnetic ion exchange resin using a combined method of chloride concentration variation and surrogate organic molecules [J]. Science of the Total Environment, 2016, 539: 165-174. |
| 21 | Hans R, Senanayake G, Dharmasiri L C S, et al. A preliminary batch study of sorption kinetics of Cr(VI) ions from aqueous solutions by a magnetic ion exchange (MIEX) resin and determination of film/pore diffusivity [J]. Hydrometallurgy, 2016, 164: 208-218. |
| 22 | Jia Y H, Ding L, Ren P Y, et al. Performances and mechanism of methyl orange and congo red adsorbed on the magnetic ion-exchange resin [J]. Journal of Chemical & Engineering Data, 2020, 65(2): 725-736. |
| 23 | 丁磊, 高阳, 贾韫翰, 等. 不同分子质量的腐殖酸对溴离子在MIEX树脂上吸附行为的影响 [J]. 过程工程学报, 2018, 18(6): 1332-1339. |
| Ding L, Gao Y, Jia Y H, et al. Effects of humic acids with different molecular weights on the adsorption behavior of bromide on MIEX resin [J]. The Chinese Journal of Process Engineering, 2018, 18(6): 1332-1339. | |
| 24 | 陈卫, 曹喆, 刘成, 等. MIEX对水中溴离子的去除效能及其影响因素 [J]. 土木建筑与环境工程, 2012, 34(3): 133-137. |
| Chen W, Cao Z, Liu C, et al. Removal efficiency and influnce factors of bromide in water by MIEX [J]. Journal of Civil, Architectural & Environmental Engineering, 2012, 34(3):133-137. | |
| 25 | 黄彬彬, 尹含双, 曹兴凯. 腐殖酸分子结构对电-Fenton性能的影响探究 [J]. 湖南大学学报(自然科学版), 2020, 47(4): 125-131. |
| Huang B B, Yin H S, Can X K. Effects of the molecular structure of humic acid on the catalytic performance of electro-Fenton [J]. Journal of Hunan University (Natural Sciences), 2020, 47(4): 125-131. | |
| 26 | 周晓霞, 孙亚兵, 朱洪标, 等. 城市景观水体中腐殖酸的臭氧氧化去除 [J]. 环境保护科学, 2010, 36(5): 10-13. |
| Zhou X X, Sun Y B, Zhu H B, et al. Removal of humic acid in urban landscape water by ozone [J]. Environmental Protection Science, 2010, 36(5): 10-13. | |
| 27 | Li A Z, Zhao X, Liu H J, et al. Characteristic transformation of humic acid during photoelectrocatalysis process and its subsequent disinfection byproduct formati potential [J]. Water Research, 2011, 45(18): 6131-6140. |
| 28 | 卓瑞双, 黄廷林, 张瑞峰, 等. 天然有机物对铁锰复合氧化膜去除氨氮的影响 [J]. 中国给水排水, 2019, 35(3): 7-12. |
| Zhuo R S, Huang T L, Zhang R F, et al. Effect of natural organic matter on ammonium removal performance of iron-manganese co-oxide film in surface water treatment [J]. China Water & Wastewater, 2019, 35(3): 7-12. | |
| 29 | 李学艳, 高乃云, 沈吉敏, 等. 水中天然有机物对粉末活性炭吸附2-MIB的影响 [J]. 给水排水, 2008, (11): 148-153. |
| Li X Y, Gao N Y, Shen J M, et al. Effect of NOM on PAC absorption of 2-MIB from raw water [J]. Water & Wastewater Engineering, 2008, (11): 148-153. | |
| 30 | 潘菲. 磁性阴离子交换树脂对腐殖酸的吸附行为与机理研究 [D]. 南京: 南京大学, 2012: 53-59. |
| Pan F. Adsorption study of humic acid on mangnetic anion exchange resin [D]. Nanjing: Nanjing University, 2012: 53-59. | |
| 31 | 张珊, 曹军, 刘成, 等. 磁性离子交换树脂对太湖原水的预处理效能 [J]. 中国给水排水, 2014, 30(5): 29-32, 36. |
| Zhang S, Cao J, Liu C, et al. Performance of MIEX for treating raw water from Taihu Lake [J]. China Water & Wastewater, 2014, 30(5): 29-32, 36. | |
| 32 | 李为兵, 陈卫, 袁哲, 等. 磁性离子交换树脂处理南方湖泊水的中试研究 [J]. 中国给水排水, 2011, 27(1): 5-7. |
| Li W B, Chen W, Yuan Z, et al. Magnetic ion exchange resin for treatment of lake water in southern China [J]. China Water & Wastewater, 2011, 27(1): 5-7. | |
| 33 | Karpinska A M, Boaventura R A R, Vítor J P V, et al. Applicability of MIEX®DOC process for organics removal from NOM laden water [J]. Environmental Ence & Pollution Research, 2013, 20(6): 3890-3899. |
| 34 | Jones K L, O’melia C R. Protein and humic acid adsorption onto hydrophilic membrane surfaces: effects of pH and ionic strength [J]. Journal of Membrane Science, 2000, 165(1): 31-46. |
| 35 | Qin X, Liu F, Wang G, et al. Adsorption of humic acid from aqueous solution by hematite: effects of pH and ionic strength [J]. Environmental Earth Sciences, 2015, 73(8): 4011-4017. |
| 36 | Lei D, Deng H, Chao W, et al. Affecting factors, equilibrium, kinetics and thermodynamics of bromide removal from aqueous solutions by MIEX resin [J]. Chemical Engineering Journal, 2012, 181/182: 360-370. |
| 37 | Wei X H, Wu Z S, Wu Z L, et al. Adsorption behaviors of atrazine and Cr(III) onto different activated carbons in single and co-solute systems [J]. Powder Technology, 2018, 329: 207-216. |
| 38 | Yin T, Wu Y, Shi P, et al. Anion-exchange resin adsorption followed by electrolysis: a new disinfection approach to control halogenated disinfection byproducts in drinking water [J]. Water Research, 2020, 168: 1-10. |
| 39 | Tang Y L, Li S Y, Zhang Y H, et al. Sorption of tetrabromobisphenol A from solution onto MIEX resin: batch and column test [J]. Journal of the Taiwan Institute of Chemical Engineers, 2014, 45(5): 2411-2471. |
| 40 | 王丹丹. MIEX树脂去除不同性能腐殖酸的特性与机制研究 [D]. 马鞍山: 安徽工业大学, 2019: 26-31. |
| Wang D D. Study on the removal characteristics and mechanism of humic acid with different properties by MIEX resin [D]. Ma'anshan: Anhui University of Technology, 2019: 26-31. | |
| 41 | Lagergren S. About the theory of so-called adsorption of soluble substances [J]. Kunliga Svenska Vetenskapsakademiens Handlingar, 1898, 24(4): 776-786. |
| 42 | Tang Y L, Wang X, Zhu L H. Removal of methyl orange from aqueous solutions with poly(acrylic acid-co-acrylamide) superabsorbent resin [J]. Polymer Bulletin, 2013, 70(3): 905-918. |
| 43 | Ho Y S, Mckay G. Pseudo-second order model for sorption processes [J]. Process Biochemistry, 1999, 34(5): 451-465. |
| 44 | 朱云华. MIEX树脂去除水源中没食子酸的特性与机理研究 [D]. 马鞍山: 安徽工业大学, 2016: 59-60. |
| Zhu Y H. The removal mechanism of gallic acid in source water by MIEX resin [D]. Ma'anshan: Anhui University of Technology, 2016: 59-60. | |
| 45 | 李炎锋, 赵威翰, 边江, 等. 城市地下长直隧道火灾近火源区长度确定 [J]. 广西大学学报(自然科学版), 2016, 41(4): 1101-1108. |
| Li Y F, Zhao W H, Bian J, et al. Length of near field of fire sourcein urban long straight underground tunnel [J]. Journal of Guangxi University (Natural Science Edition), 2016, 41(4): 1101-1108. | |
| 46 | Ding L, Zhu Y H, Jin X P, et al. Removal of chlorite from aqueous solution by MIEX resin [J]. Desalination & Water Treatment, 2017, 77: 264-273. |
| 47 | Langmuir I. The constitution and fundamental properties of solids and liquids [J]. Journal of the American Chemical Society, 1916, 38(11): 2221-2295. |
| 48 | Sips R. Combined form of Langmuir and Freundlich equations [J]. Journal of Chemical Physics, 1948, 16: 490-495. |
| 49 | Freundlich H. Zeitschrift fur physikalische chemie (Leipzig) [M]. Leipzig: Akademische Verlagsgesellschaft Becker & Erler Kom, 1943: 385-470. |
| 50 | 骆晓琳. 利用交联法制备改性腐植酸基吸附剂及其性能研究 [D]. 西安: 西安工程大学, 2018: 39-43. |
| Luo X L. Preparation of modified humic acid based adsorbent by cross-linking and the research of its properties [D]. Xi΄an: Xi΄an Polythchnic University, 2018: 39-43. |
| [1] | 张继欢 张晋玮 武文龙 林嗣强 李燕 丁磊. 碳酸氢钠改性生物炭的制备及其吸附水中卡马西平的机理分析[J]. 过程工程学报, 2024, 24(9): 1106-1119. |
| [2] | 邱明俊 李小敏 尚华 杨江峰 李晋平. 基于低浓度甲烷富集的变压吸附工艺流程优化模拟[J]. 过程工程学报, 2024, 24(8): 884-893. |
| [3] | 陈寿天宝 张凯 许振良 程亮 徐孙杰 魏永明. 电子级HEA制备中痕量钠钾离子吸附及过程模拟[J]. 过程工程学报, 2024, 24(7): 843-851. |
| [4] | 武文龙 高俣辰 张晋玮 李凌 李燕 曾宇晨 杨春 陆嘉鹏 丁磊. 紫外光协同磁性离子交换树脂活化过硫酸盐去除水中腐殖酸的效能与机理[J]. 过程工程学报, 2024, 24(5): 566-579. |
| [5] | 汪前雨 张玉明 崔彦斌. 磁性活性炭制备及其在水处理中的研究进展[J]. 过程工程学报, 2024, 24(3): 259-272. |
| [6] | 谭雅婕 胡宪伟 杨酉坚 刘爱民 石忠宁 汤帅 王兆文. 氟化氢在氧化铝表面吸附机理的Al8O12团簇模型量子化学计算[J]. 过程工程学报, 2024, 24(2): 218-226. |
| [7] | 向波 刘晨明 曹仁强 段锋 李玉平. 大孔树脂对镍钴萃取废水中有机物的吸附性能[J]. 过程工程学报, 2024, 24(2): 227-237. |
| [8] | 刘旭东 李玉然 彭书雯 刘利 徐文青 朱廷钰. 高炉煤气中H2S在羟基氧化铁上的吸附氧化机理研究[J]. 过程工程学报, 2024, 24(12): 1417-1424. |
| [9] | 许丽 耿娜 刘文 田京雷 朱廷钰 姚明水 郭旸旸. 含硫烟气对MFM-136捕集CO2的影响及烟气组分共吸附机理研究[J]. 过程工程学报, 2024, 24(12): 1425-1434. |
| [10] | 胡义明 曹阳 黄洋 杨诚 高翔鹏 李明阳. 二胺类药剂对霓石絮凝沉降性能的影响及吸附机理研究[J]. 过程工程学报, 2024, 24(11): 1318-1325. |
| [11] | 徐颖 姚鑫毅 宋永红 孙一平 邹晶晶 郭春彬. 煤气化渣改性工艺及吸附Cd2+性能[J]. 过程工程学报, 2024, 24(1): 47-57. |
| [12] | 殷慧卿 武少杰 李明阳 龙红明 王松月 邱志新 高翔鹏. CCS-DETA凝胶球的制备及其对甲基橙的吸附性能[J]. 过程工程学报, 2023, 23(4): 590-601. |
| [13] | 王思栋 王留洋 冯雪 张松平 张万忠 罗坚. 静电耦合亲和层析纯化人血清白蛋白的研究[J]. 过程工程学报, 2023, 23(11): 1599-1607. |
| [14] | 陆炫彤 赵金 邓春. 丙烷脱氢反应气分离提纯丙烯和氢气过程严格模拟与能效评估[J]. 过程工程学报, 2023, 23(1): 144-153. |
| [15] | 赵晓腾 周新涛 罗中秋 韦宇 兰雄 陆艳. 二氧化钛基复合材料对常见染料的去除性能及其机理研究进展[J]. 过程工程学报, 2022, 22(9): 1169-1180. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||