

The Chinese Journal of Process Engineering ›› 2021, Vol. 21 ›› Issue (7): 836-846.DOI: 10.12034/j.issn.1009-606X.220175
• Process & Technology • Previous Articles Next Articles
Xiaoliang LI1,2( ), Guocai TIAN1,2(
), Guocai TIAN1,2( )
)
Received:2020-06-08
Revised:2020-09-03
Online:2021-07-28
Published:2021-07-27
Contact:
Guocai TIAN 1609488137@qq.com;tiangc01@163.com
通讯作者:
田国才 1609488137@qq.com;tiangc01@163.com
作者简介:李小亮(1993-),男,江西省吉安市人,硕士研究生,冶金工程专业,E-mail: 1609488137@qq.com基金资助:CLC Number:
Xiaoliang LI, Guocai TIAN. First-principles calculation of adsorption mechanism of hydrochloric acid on chalcopyrite surface[J]. The Chinese Journal of Process Engineering, 2021, 21(7): 836-846.
李小亮, 田国才. 盐酸在黄铜矿表面吸附机制的第一性原理计算[J]. 过程工程学报, 2021, 21(7): 836-846.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.jproeng.com/EN/10.12034/j.issn.1009-606X.220175
| Parameter | This work | PWscf[ | VASP[ | Siesta[ | Experimental values[ |
|---|---|---|---|---|---|
| a=b | 0.5256 | 0.5263 | 0.5279 | 0.5277 | 0.5289 |
| c | 1.0395 | 1.0362 | 1.0364 | 1.0447 | 1.0423 |
| Fe-S | 0.2229 | 0.2241 | 0.2257 | 0.2250 | 0.2257 |
| Cu-S | 0.2307 | 0.2293 | 0.2287 | 0.2300 | 0.2302 |
| Fe-Fe | 0.3696 | 0.3693 | - | - | 0.3713 |
| Cu-Cu | 0.3696 | 0.3693 | - | - | 0.3713 |
| Fe-Cu | 0.3716 | 0.3721 | - | - | 0.3740 |
| S-S | 0.3622 | 0.3659 | - | - | 0.3685 |
Table 1 Geometrical parameters of the chalcopyrite bulk after optimized
| Parameter | This work | PWscf[ | VASP[ | Siesta[ | Experimental values[ |
|---|---|---|---|---|---|
| a=b | 0.5256 | 0.5263 | 0.5279 | 0.5277 | 0.5289 |
| c | 1.0395 | 1.0362 | 1.0364 | 1.0447 | 1.0423 |
| Fe-S | 0.2229 | 0.2241 | 0.2257 | 0.2250 | 0.2257 |
| Cu-S | 0.2307 | 0.2293 | 0.2287 | 0.2300 | 0.2302 |
| Fe-Fe | 0.3696 | 0.3693 | - | - | 0.3713 |
| Cu-Cu | 0.3696 | 0.3693 | - | - | 0.3713 |
| Fe-Cu | 0.3716 | 0.3721 | - | - | 0.3740 |
| S-S | 0.3622 | 0.3659 | - | - | 0.3685 |
| Parameter | This work | Siesta[ | VASP[ | PWscf[ |
|---|---|---|---|---|
| S-S | 0.220 | 0.223 | 0.2242 | 0.2158 |
| Fe-S | 0.214 | 0.224 | 0.2182 | 0.2319 |
| Cu-S | 0.235 | 0.230 | 0.2359 | 0.2326 |
Table 2 Reconstructed chalcopyrite (001)-S surface
| Parameter | This work | Siesta[ | VASP[ | PWscf[ |
|---|---|---|---|---|
| S-S | 0.220 | 0.223 | 0.2242 | 0.2158 |
| Fe-S | 0.214 | 0.224 | 0.2182 | 0.2319 |
| Cu-S | 0.235 | 0.230 | 0.2359 | 0.2326 |
| Adsorption site for H+ | ΔΕ/(kcal/mol) | Distance of Fe-Cl/nm | Siesta[ |
|---|---|---|---|
| 1 | -19.4 | 0.232 | -19.2 |
| 2 | -19.5 | 0.231 | -19.3 |
| 3 | -14.4 | 0.227 | -14.5 |
| 4 | -12.8 | 0.230 | -11.9 |
| 5 | -20.9 | 0.230 | -21.2 |
| 6 | -20.0 | 0.232 | -21.0 |
| 7 | -13.5 | 0.230 | -14.6 |
| 8 | -10.6 | 0.229 | - |
Table 3 The adsorption energy and Fe-Cl bond length considering different adsorption sites for H+ in the dissociation of hydrochloric acid
| Adsorption site for H+ | ΔΕ/(kcal/mol) | Distance of Fe-Cl/nm | Siesta[ |
|---|---|---|---|
| 1 | -19.4 | 0.232 | -19.2 |
| 2 | -19.5 | 0.231 | -19.3 |
| 3 | -14.4 | 0.227 | -14.5 |
| 4 | -12.8 | 0.230 | -11.9 |
| 5 | -20.9 | 0.230 | -21.2 |
| 6 | -20.0 | 0.232 | -21.0 |
| 7 | -13.5 | 0.230 | -14.6 |
| 8 | -10.6 | 0.229 | - |
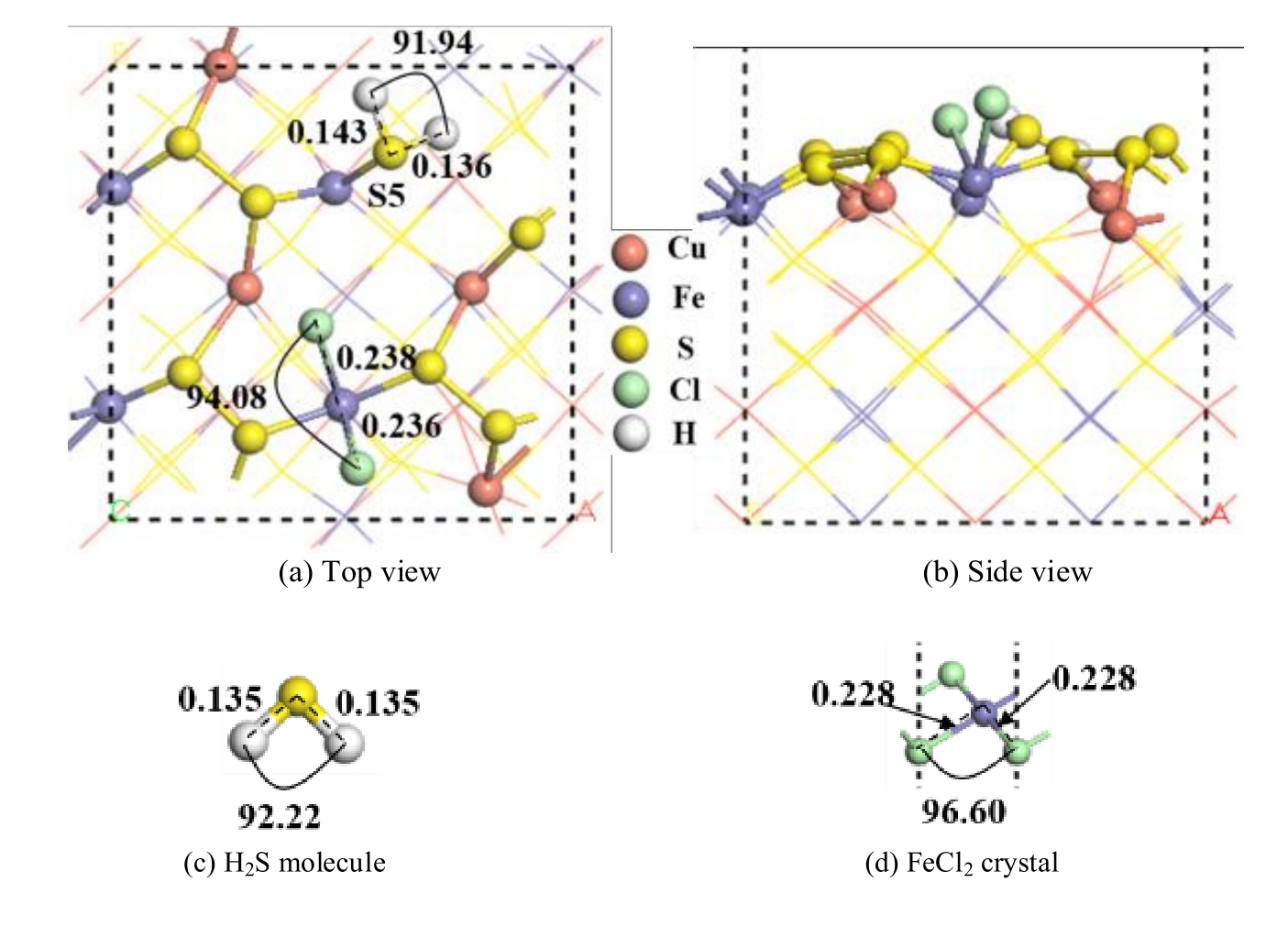
Fig.9 The most stable configuration of two hydrochloric acid molecules dissociated and adsorbed on chalcopyrite (001)-S surface and H2S molecule and FeCl2 crystal optimized (unit: nm)
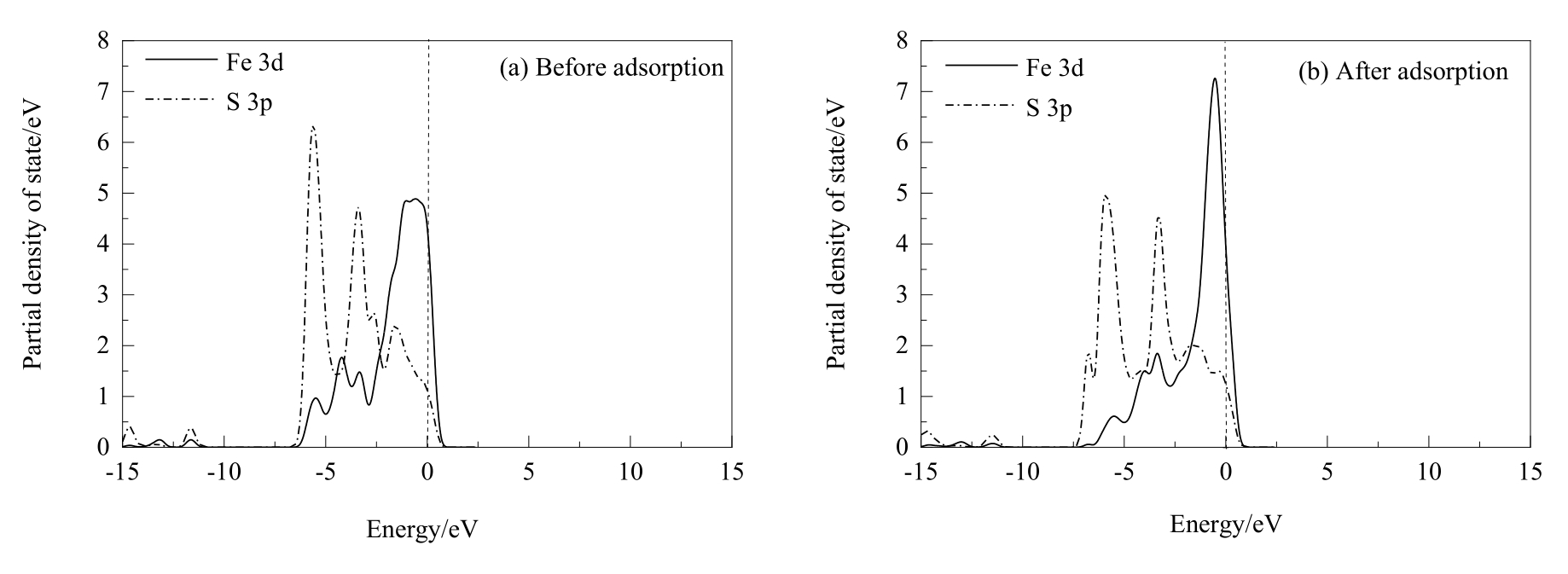
Fig.11 PDOS analysis of Fe and S atoms before and after two hydrochloric acid molecules dissociative adsorption on chalcopyrite (001)-S surface (the dash lines represent Fermi level)
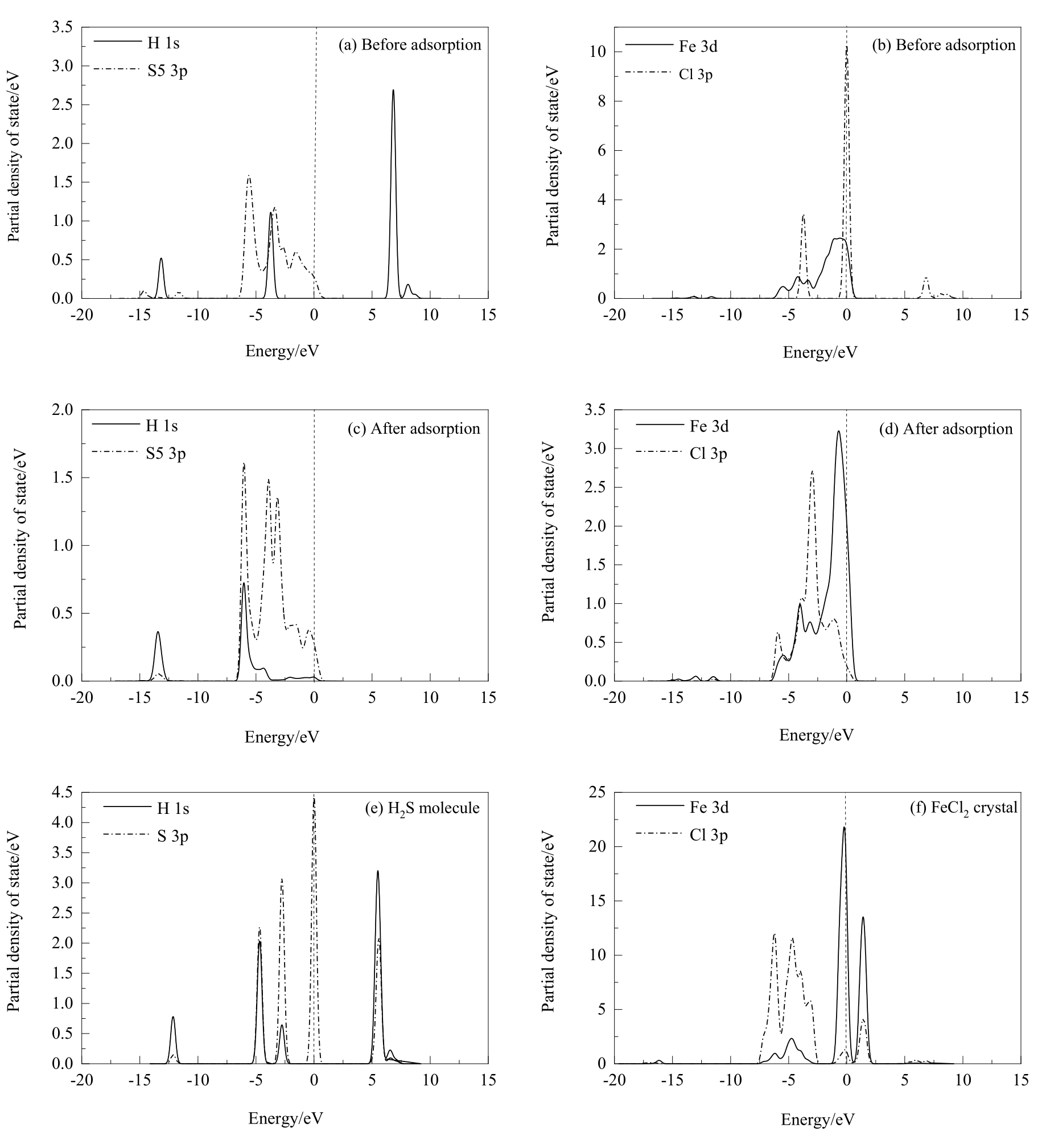
Fig.12 PDOS analysis of hydrochloric acid before and after dissociative adsorption on chalcopyrite (001)-S surface and the H2S molecule and FeCl2 crystal optimized
| Atom | Adsorption | S orbital, s/e | P orbital, p/e | D orbital, d/e | Total | Charge/e |
|---|---|---|---|---|---|---|
| H | Before | 0.66 | 0.00 | 0.00 | 0.66 | 0.34 |
| H | After | 0.91 | 0.00 | 0.00 | 0.91 | 0.09 |
| Cl | Before | 1.93 | 5.41 | 0.00 | 7.34 | -0.34 |
| Cl | After | 1.95 | 5.35 | 0.00 | 7.29 | -0.29 |
| S5 | Before | 1.87 | 4.27 | 0.00 | 6.14 | -0.14 |
| S5 | After | 1.85 | 4.38 | 0.00 | 6.23 | -0.23 |
| Fe | Before | 0.36 | 0.50 | 6.94 | 7.79 | 0.21 |
| Fe | After | 0.37 | 0.50 | 6.91 | 7.77 | 0.23 |
Table 4 Mulliken charge population of atoms before and after dissociative adsorption of hydrochloric acid on chalcopyrite (001)-S surface
| Atom | Adsorption | S orbital, s/e | P orbital, p/e | D orbital, d/e | Total | Charge/e |
|---|---|---|---|---|---|---|
| H | Before | 0.66 | 0.00 | 0.00 | 0.66 | 0.34 |
| H | After | 0.91 | 0.00 | 0.00 | 0.91 | 0.09 |
| Cl | Before | 1.93 | 5.41 | 0.00 | 7.34 | -0.34 |
| Cl | After | 1.95 | 5.35 | 0.00 | 7.29 | -0.29 |
| S5 | Before | 1.87 | 4.27 | 0.00 | 6.14 | -0.14 |
| S5 | After | 1.85 | 4.38 | 0.00 | 6.23 | -0.23 |
| Fe | Before | 0.36 | 0.50 | 6.94 | 7.79 | 0.21 |
| Fe | After | 0.37 | 0.50 | 6.91 | 7.77 | 0.23 |
| 1 | Al-Harahsheh M, Kingman S, Al-Harahsheh A. Ferric chloride leaching of chalcopyrite: synergetic effect of CuCl2 [J]. Hydrometallurgy, 2008, 91(1/2/3/4): 89-97. |
| 2 | Córdoba E M, Muñoz J A, Blázquez M L, et al. Leaching of chalcopyrite with ferric ion. part I: general aspects [J]. Hydrometallurgy, 2008, 93(3/4): 81-87. |
| 3 | Klauber C. A critical review of the surface chemistry of acidic ferric sulphate dissolution of chalcopyrite with regards to hindered dissolution [J]. International Journal of Mineral Processing, 2008, 86(1/2/3/4): 1-17. |
| 4 | Hiroyoshi N, Miki H, Hirajima T, et al. Enhancement of chalcopyrite leaching by ferrous ions in acidic ferric sulfate solutions [J]. Hydrometallurgy, 2001, 60(3): 185-197. |
| 5 | Acero P, Cama J, Ayora C. Kinetics of chalcopyrite dissolution at pH=3 [J]. European Journal of Mineralogy, 2007, 19(2): 173-182. |
| 6 | Li Y, Yao Y, Wang B, et al. New insights into chalcopyrite leaching enhanced by mechanical activation [J]. Hydrometallurgy, 2019, 189: 105131-105137. |
| 7 | Córdoba E M, Muñoz J A, Blázquez M L, et al. Leaching of chalcopyrite with ferric ion. part II: effect of redox potential [J]. Hydrometallurgy, 2008, 93(3/4): 88-96. |
| 8 | Hackl R P, Dreisinger D B, Peters E, et al. Passivation of chalcopyrite during oxidative leaching in sulfate media [J]. Hydrometallurgy, 1995, 39(1/2/3): 25-48. |
| 9 | Shin D, Ahn J, Lee J. Kinetic study of copper leaching from chalcopyrite concentrate in alkaline glycine solution [J]. Hydrometallurgy, 2019, 183: 71-78. |
| 10 | Ruiz M C, Montes K S, Padilla R. Chalcopyrite leaching in sulfate-chloride media at ambient pressure [J]. Hydrometallurgy, 2011, 109(1/2): 37-42. |
| 11 | Li J, Kawashima N, Kaplun K, et al. Chalcopyrite leaching: the rate controlling factors [J]. Geochimica et Cosmochimica Acta, 2010, 74(10): 2881-2893. |
| 12 | Hall S R, Stewart J M. The crystal structure refinement of chalcopyrite, CuFeS2 [J]. Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry, 1973, 29(3): 579-585. |
| 13 | Harmer S L, Pratt A R, Nesbitt W H, et al. Sulfur species at chalcopyrite (CuFeS2) fracture surfaces [J]. American Mineralogist, 2004, 89(7): 1026-1032. |
| 14 | Klauber C. Fracture-induced reconstruction of a chalcopyrite (CuFeS2) surface [J]. Surface and Interface Analysis, 2003, 35(5): 415-428. |
| 15 | Harmer S L, Thomas J E, Fornasiero D, et al. The evolution of surface layers formed during chalcopyrite leaching [J]. Geochimica et Cosmochimica Acta, 2006, 70(17): 4392-4402. |
| 16 | Petrovic S J, Bogdanovic G D, Antonijevic M M. Leaching of chalcopyrite with hydrogen peroxide in hydrochloric acid solution [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(7): 1444-1455. |
| 17 | Lu Z Y, Jeffrey M I, Lawson F. An electrochemical study of the effect of chloride ions on the dissolution of chalcopyrite in acidic solutions [J]. Hydrometallurgy, 2000, 56(2): 145-155. |
| 18 | Velásquez-Yévenes L, Nicol M, Miki H. The dissolution of chalcopyrite in chloride solutions: part 1: the effect of solution potential [J]. Hydrometallurgy, 2010, 103(1/2/3/4): 108-113. |
| 19 | Velásquez-Yévenes L, Miki H, Nicol M. The dissolution of chalcopyrite in chloride solutions: part 2: effect of various parameters on the rate [J]. Hydrometallurgy, 2010, 103(1/2/3/4): 80-85. |
| 20 | Nicol M, Miki H, Velásquez-Yévenes L. The dissolution of chalcopyrite in chloride solutions: part 3: mechanisms [J]. Hydrometallurgy, 2010, 103(1/2/3/4): 86-95. |
| 21 | Miki H, Nicol M. The dissolution of chalcopyrite in chloride solutions: part IV: the kinetics of the auto-oxidation of copper (I) [J]. Hydrometallurgy, 2011, 105(3/4): 246-250. |
| 22 | Watling H R. Chalcopyrite hydrometallurgy at atmospheric pressure: review of acidic chloride process options [J]. Hydrometallurgy, 2014, 146: 96-110. |
| 23 | Martínez-Gómez V J, Fuentes-Aceituno J C, Pérez-Garibay R, et al. A study of the electro-assisted reductive leaching of a chalcopyrite concentrate in HCl solutions. part I: kinetic behavior and nature of the chalcopyrite reduction [J]. Hydrometallurgy, 2018, 181: 195-205. |
| 24 | Biegler T. Reduction kinetics of a chalcopyrite electrode surface [J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1977, 85(1): 101-106. |
| 25 | Biegler T, Swift D A. The electrolytic reduction of chalcopyrite in acid solution [J]. Journal of Applied Electrochemistry, 1976, 6(3): 229-235. |
| 26 | Tesinsky M, Balaz P. Copper leaching from chalcopyrite: mechanochemical approach [J]. Inżynieria Mineralna, 2017, 18(1): 1-5. |
| 27 | 陈建华, 王进明, 龙贤灏, 等. 硫化铜矿物电子结构的第一性原理研究 [J] 中南大学学报(自然科学版), 2011, 42(12): 16-21. |
| Chen J H, Wang J M, Long X H, et al. First-principle theory on electronic structure of copper sulfides [J]. Journal of Central South University (Science and Technology), 2011, 42(12): 16-21. | |
| 28 | de Oliveira C, de Lima G F, de Abreu H A, et al. Reconstruction of the chalcopyrite surfaces: a DFT study [J]. The Journal of Physical Chemistry C, 2012, 116(10): 6357-6366. |
| 29 | de Lima G F, de Oliveira C, de Abreu H A, et al. Water adsorption on the reconstructed (001) chalcopyrite surfaces [J]. The Journal of Physical Chemistry C, 2011, 115(21): 10709-10717. |
| 30 | de Lima G F, de Oliveira C, de Abreu H A, et al. Sulfuric and hydrochloric acid adsorption on the reconstructed sulfur terminated (001) chalcopyrite surface [J]. International Journal of Quantum Chemistry, 2012, 112(19): 3216-3222. |
| 31 | Perdew J P, Burke K, Ernzerhof M. Generalized gradient approximation made simple [J]. Physical Review Letters, 1996, 77(18): 3865-3868. |
| 32 | de Oliveira C, Duarte H A. Disulphide and metal sulphide formation on the reconstructed (001) surface of chalcopyrite: a DFT study [J]. Applied Surface Science, 2010, 257(4): 1319-1324. |
| 33 | Li K, Zhao Y, Zhang P, et al. Combined DFT and XPS investigation of iodine anions adsorption on the sulfur terminated (001) chalcopyrite surface [J]. Applied Surface Science, 2016, 390: 412-421. |
| 34 | Li Y, Chandra A P, Gerson A R. Scanning photoelectron microscopy studies of freshly fractured chalcopyrite exposed to O2 and H2O [J]. Geochimica et Cosmochimica Acta, 2014, 133: 372-386. |
| 35 | Li Y, Kawashima N, Li J, et al. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite [J]. Advances in Colloid and Interface Science, 2013, 197: 1-3. |
| 36 | Benedek R, Thackeray M M, Low J J, et al. Simulation of aqueous dissolution of lithium manganate spinel from first principles [J]. Journal of Physical Chemistry C, 2012, 116(6): 4050-4059. |
| 37 | Leung K. First-principles modeling of Mn(II) migration above and dissolution from Li x Mn2O4 (001) surfaces [J]. Chemistry of Materials, 2017, 29(6): 2550-2562. |
| 38 | Stack A G, Raiteri P, Gale J D, et al. Accurate rates of the complex mechanisms for growth and dissolution of minerals using a combination of rare-event theories [J]. Journal of the American Chemical Society, 2012, 134(1): 11-14. |
| 39 | Ionescu A, Allouche A, Aycard J P, et al. Study of γ-alumina surface reactivity: adsorption of water and hydrogen sulfide on octahedral aluminum sites [J]. Journal of Physical Chemistry B, 2002, 106(36): 9359-9366. |
| 40 | Lectez S, Roques J, Salanne M, et al. Car-Parrinello molecular dynamics study of the uranyl behaviour at the gibbsite/water interface [J]. Journal of Chemical Physics, 2012, 137(15): 154705-154713. |
| [1] | Shuang QIN Jianjun FANG Haiyang HE Zhilian QIU Liguo PENG Shiqin DONG. The role and mechanism of dihydromyricetin in the flotation of chalcopyrite and galena [J]. The Chinese Journal of Process Engineering, 2024, 24(11): 1335-1343. |
| [2] | Yusheng ZHOU Guanzhou QIU Jianfa JING Fuqiang ZHENG Shuai WANG Feng CHEN Yufeng GUO. A novel process for preparation Ti-rich material from modified electric furnace titanium slag by phase deconstruction method [J]. The Chinese Journal of Process Engineering, 2022, 22(5): 651-659. |
| [3] | Shuai SUN Hongqian SUN Jing SONG Jingkui QU Yong WANG Tao QI. Experimental and model analysis of hydrochloric acid recovery by dynamic diffusion dialysis [J]. Chin. J. Process Eng., 2021, 21(1): 57-63. |
| [4] | Dan LI Desheng CHEN Guozhi ZHANG Hongxin ZHAO Tao QI Weijing WANG . Separation of Vanadium(IV) from Iron(II) in Hydrochloric Acid Solution by Solvent Extraction with P507 [J]. Chin. J. Process Eng., 2017, 17(6): 1182-1187. |
| [5] | Guocai TIAN Junxian HU Futing ZI. Research Advances in Applications of Oxidants in Oxidation Leaching of Chalcopyrite [J]. Chin. J. Process Eng., 2017, 17(4): 664-676. |
| [6] | XIAO Wan-hai ZHAO Hong-xin SONG Ning CHEN De-sheng LIU Ya-hui WANG Li-na Li-Na QI Tao. Leaching of Fe, V and Ti from Pre-reduced Vanadium-bearing Titanomagnetite Bulk Concentrate with HCl and H2SO4 [J]. Chin. J. Process Eng., 2016, 16(5): 737-743. |
| [7] | SHAO Da-wei LIU Ya-hui WANG Wei-jing CAO Cheng-bo QI Tao. Leaching Kinetics of Reductively Roasted Slag with HCl [J]. Chin. J. Process Eng., 2016, 16(4): 577-583. |
| [8] | LIU Peng-fei ZHANG Yi-fei YOU Shao-wei BO Jing JIANG Xiao-duo. Recovery of Valuable Elements in Jaroaite Residue by Hot Acid Leaching [J]. Chin. J. Process Eng., 2016, 16(4): 584-589. |
| [9] | XIE Zi-nan CHEN Qian-lin ZHAO Li-jun. Extraction of Rare Earth Ions by Emulsion Liquid Membrane from Acidic Leaching Solution of Phosphorus-containing Ore [J]. , 2013, 13(2): 197-201. |
| [10] | WANG Bao-quan GUO Qiang QU Jing-kui QI Tao. Optimization of Conditions in Atmospheric Acid Leaching of the Water-leached Residue of Limonitic Laterite after Alkali-roasting [J]. , 2012, 12(3): 420-426. |
| [11] | GENG Jie CHEN Jian-ding MA Xin-sheng. Cationic Modification on the Surface of Fly Ash Fibres Used in Papermaking [J]. , 2010, 10(6): 1212-1216. |
| [12] | FU Nian-xin Iwasaki Iwao Tamagawa Takeo Kobayashi Mikio. Extraction Process of Copper from Chalcopyrite Concentrate by Low-temperature Chlorination-Selective Oxidation [J]. , 2010, 10(6): 1138-1142. |
| [13] | LIANG Xiang-bo WU Pan LV Li LIANG Bin LI Chun. Effect of Preoxidation on Hydrochloric Acid Leaching of Panzhihua Ilmenite [J]. , 2010, 10(5): 939-943. |
| [14] | LIU Dai-yun WEI Zhen-dong; HUA Mei; LIU Hui-yong. Cyclic Voltammetry Analysis of Bioleaching of Low Grade Chalcopyrite by Thermophilic Acidianus brierleyi [J]. , 2010, 10(5): 927-932. |
| [15] | FU Nian-xin Iwasaki Iwao Tamagawa Takeo Kobayashi Mikio. Selective Oxidation Behavior of Chlorides Produced by Chlorination of Chalcopyrite Concentrate [J]. , 2009, 9(6): 1080-1084. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||